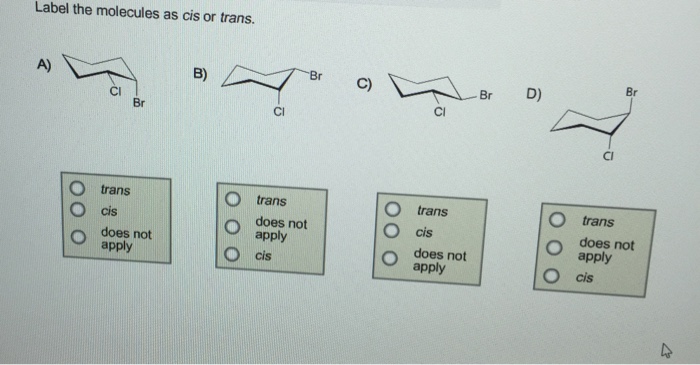
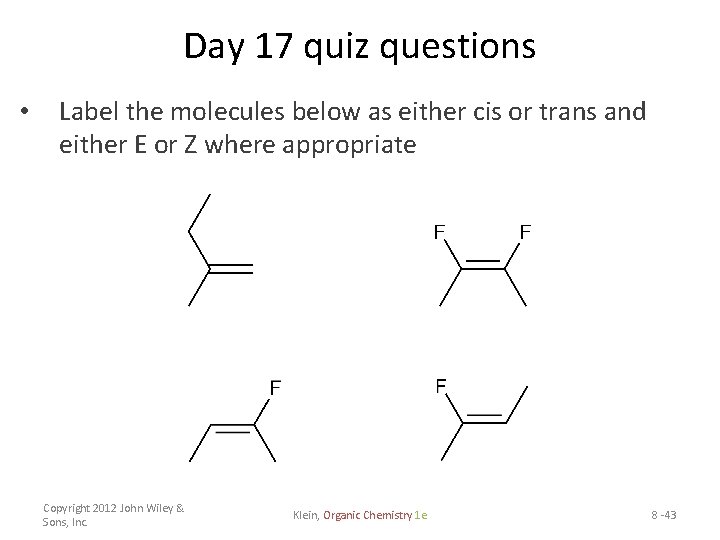
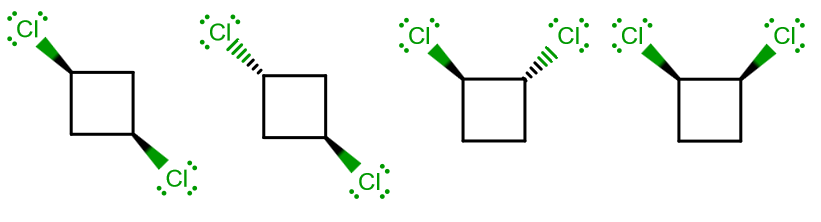
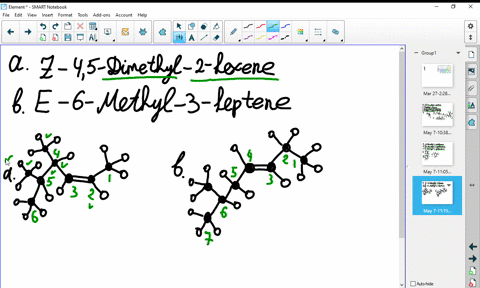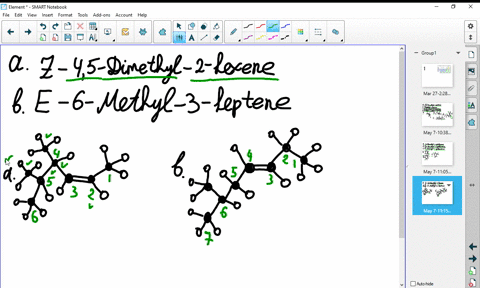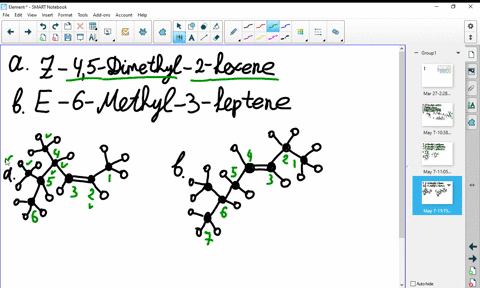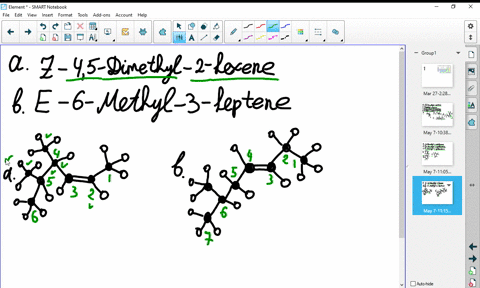
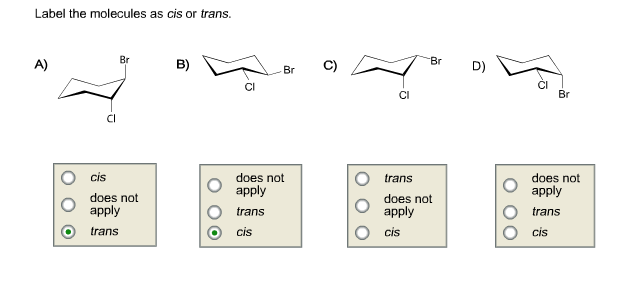
44 label the molecules as cis or trans.
The cis and trans geometric isomers of a compound can have different ... For each molecule, indicate whether cis-trans isomers exist. If they do, draw the two isomers and label them as cis and trans. a. CH3 - CH2 -CH=CH, b. CH3-CH2-CH=CH c. 2-Pentene d. 1,2-Dichloroethene Label the compound as cis or trans. Be sure to answer all parts. For each compound... Label the compound as cis or trans. Be sure to answer all parts. Solved Label the molecules as cis or trans. Trans cis | Chegg.com Question: Label the molecules as cis or trans. Trans cis does not apply trans does not apply cis trans cis does not apply trans does not apply cis. This problem has been solved! See the answer See the answer See the answer done loading. Show transcribed image text Expert Answer.
Solved Label the molecules as cis or trans. | Chegg.com Label the molecules as cis or trans. Question: Label the molecules as cis or trans. This problem has been solved! See the answer See the answer See the answer done loading. Show transcribed image text Expert Answer. Who are the experts? Experts are tested by Chegg as specialists in their subject area. We review their content and use your ...
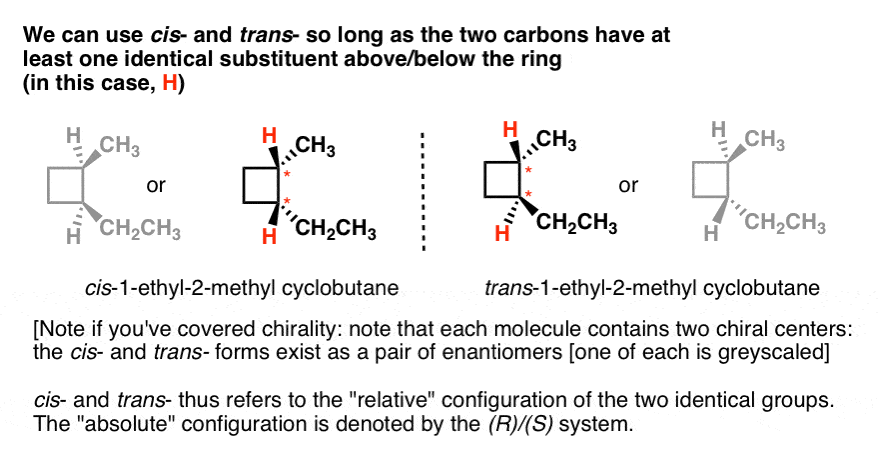
Label the molecules as cis or trans.
Geometric Isomerism Cis- and Trans- Mean in Chemistry - ThoughtCo In geometrical isomer nomenclature, the prefix cis- and trans- are used to identify which side of the double bond the similar atoms are found. The cis- prefix is from the Latin meaning "on this side". In this case, the chlorine atoms are on the same side of the carbon-carbon double bond. This isomer is called cis-1,2-dichloroethene. Which of the following molecules can exist as cis and trans ... - OneClass 14. Which of the following molecules have NO cis/trans isomers? If there is a cis/trans isomer, draw and label both isomers as E and Z. 15. Give the mechanism for the following reaction and label the rate limiting step + H20 heat 16. Predict the major product of both reactions and label products as Hoffman or Zaitsev's product. Study Chapter 4 Flashcards | Quizlet Label each pair of compounds below as: a. conformational isomers b. stereoisomers ... c. cis-trans isomers d. both b and c e. a, b and c ... a. identical molecules b. constitutional isomers c. stereoisomers d. different molecules. b. A: axial B: equatorial. Consider the two methyl groups indicated with letters in the following molecular model ...
Label the molecules as cis or trans.. How can you identify cis and trans isomers? + Example - Socratic.org Explanation: Both of the isomers have exactly the same atoms joined up in exactly the same order .It means that the Van der Waals dispersion forces between the molecules will be identical in both cases. The difference between the two is that the 1) Cis I somer is polar whereas the T rans I somer is non − polar. Answered: Label the molecules as cis or trans. Br… | bartleby Label the molecules as cis or trans. Br B) CI 2 Br 6 Br D) -0 Br Question Transcribed Image Text:Label the molecules as cis or trans. Br B) CI does not apply trans cis Br trans cis does not apply 6 Br cis does not apply trans D) -G Br does not apply trans cis Expert Solution Want to see the full answer? Check out a sample Q&A here See Solution Cis and Trans - Organic Chemistry | Socratic The cis-trans definition is unambiguous only when you have two different groups on one of the alkene carbons and the same two groups on the other carbon, as in but-2-ene. Then the two identical methyl groups are either cis or trans to each other, and the two identical hydrogen atoms are either cis or trans to each other. Label the molecules as cis or trans. Trans cis does not apply trans ... Label the molecules as cis or trans. does not apply trans cis trans cis does not apply does not apply trans cis trans cis does not apply Posted one year ago. Q: 1. For each molecule, indicate whether cis-trans isomers exist. If they do, draw the two isomers and label them as cis and trans. 2.
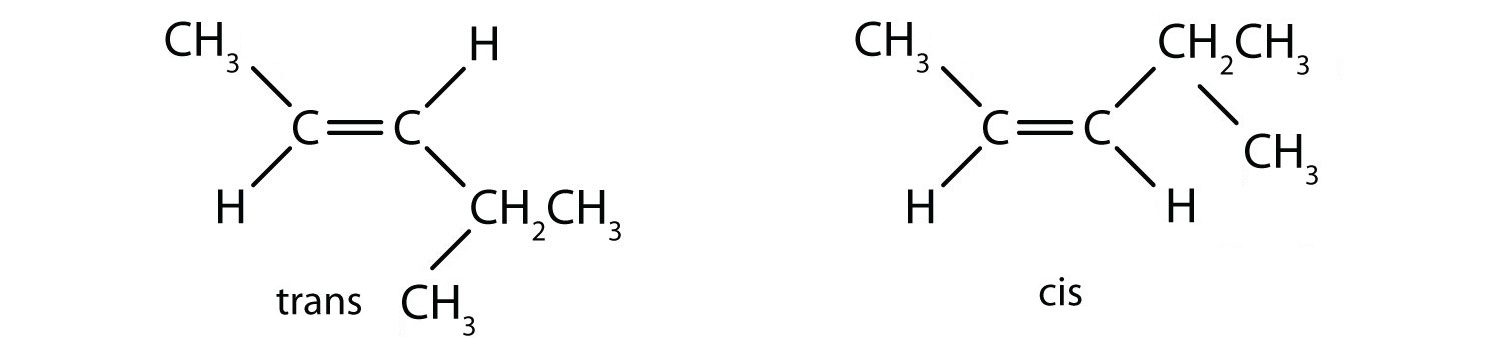
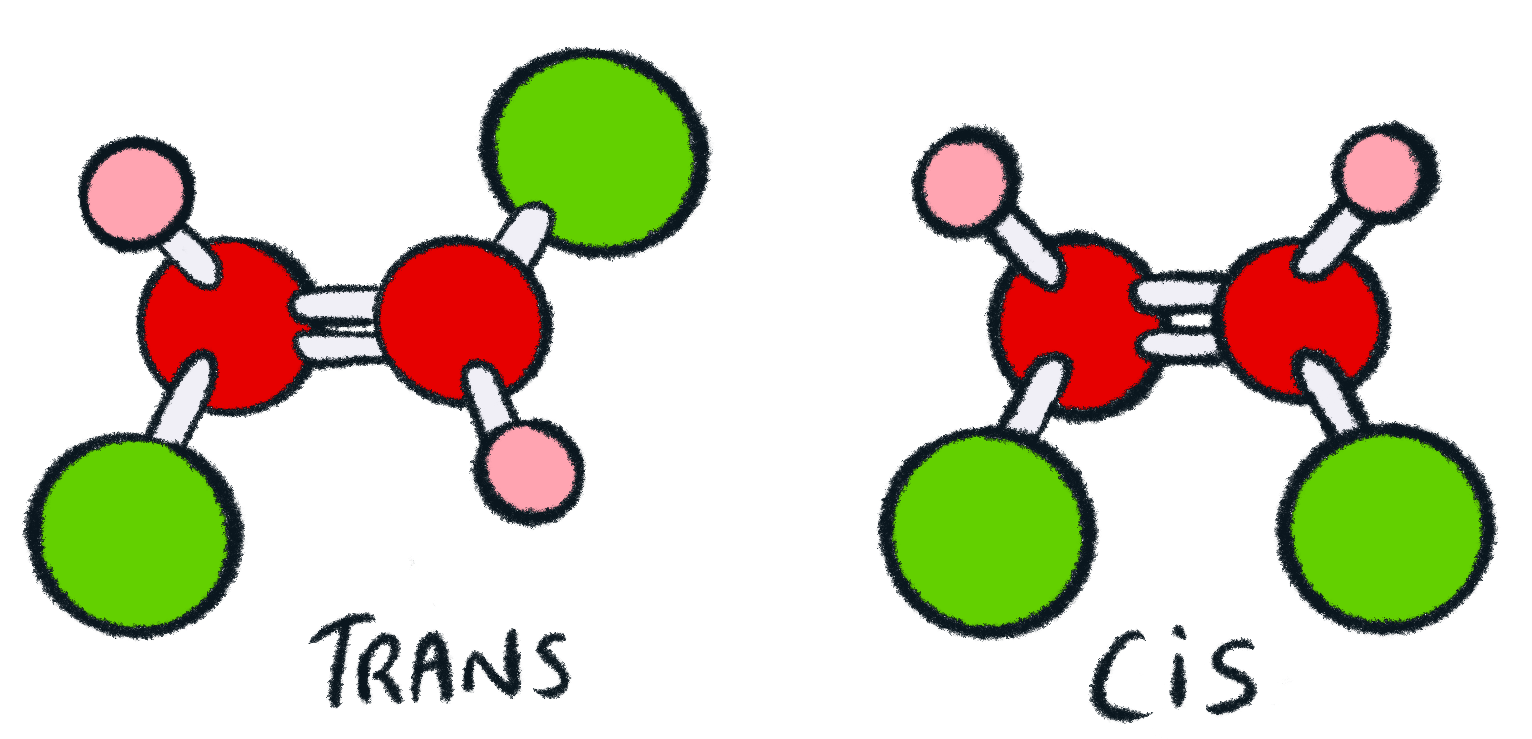
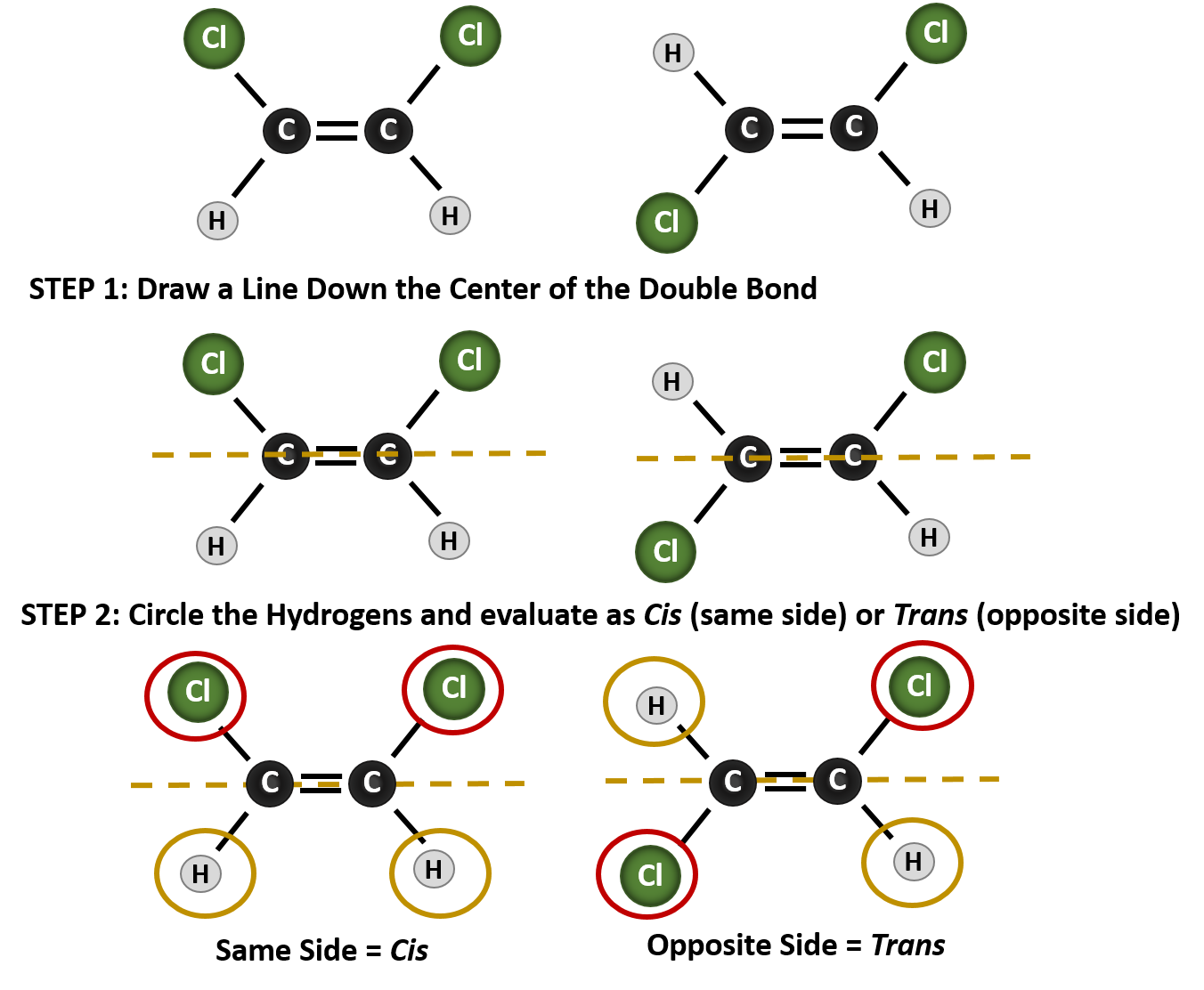
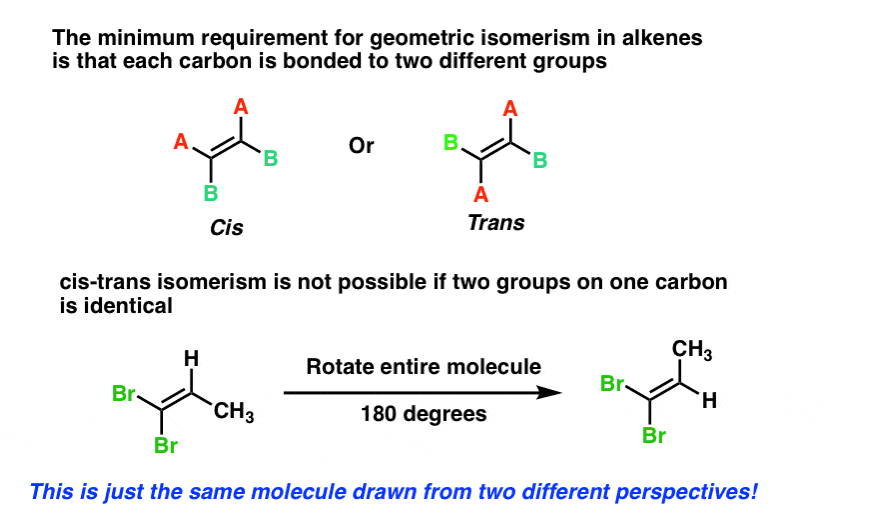
geometric (cis / trans) isomerism - chemguide Explains what geometric (cis / trans) isomerism is and how you recognise the possibility of it in a molecule. ... By contrast, although there will still be polar bonds in the trans isomers, overall the molecules are non-polar. The slight charge on the top of the molecule (as drawn) is exactly balanced by an equivalent charge on the bottom. ... Cis and Trans Alkenes - Chemistry Steps The origin of the cis and trans isomerism is the "locked" feature of the double. It locked because there is no rotation around the double bond and this, in turn, means that we cannot switch the orientation of the groups on the double bond. Recall that there is a free rotation about sigma bonds and that is the origin of conformers: Cis-Trans Isomers (Geometric Isomers) - Lardbucket.org The isomer with the two Cl atoms on opposite sides of the molecule is the trans isomer. An isomer in which two substituent groups are attached to opposite sides of a double bond or ring in a molecule. (Latin trans, meaning "across") and is named trans -1,2-dichloroethene. These two compounds are cis-trans isomers (or geometric isomers) Answered: 15. Identify the alkenes below as cis,… | bartleby Science Chemistry Q&A Library 15. Identify the alkenes below as cis, trans, E or Z. Not all the labels will be used. Some molecules may have more than one correct answer. CI Br. Br. 15. Identify the alkenes below as cis, trans, E or Z. Not all the labels will be used.
Cis-Trans Isomers - Definition, Detailed Explanation with Examples - BYJUS Maleic acid is the cis isomer and fumaric acid is the trans isomer. Elaidic acid and oleic acid are cis-trans isomers. The former is solid at room temperature (melting point = 43 o C) and the latter is found to be liquid, with a melting point of 13.4 o What are Cis and Trans Double Bonds? That's Simple! - Quirky Science If a molecule has more than one double bond, the molecule can have both cis and trans bonds. For example, 3,4-dimethyl-hexa-2,4-diene, with two, has one cis-double bond and one trans-double bond (see topmost image). The difference between cis and trans is not merely of intellectual value. Life chemistry requires very specific chemical structures. Answered: Label the following molecules as in the… | bartleby Solution for Label the following molecules as in the cis or trans configuration? Answered: Q1:Drawand label the Cis-and… | bartleby Answered: Q1:Drawand label the Cis-and… | bartleby. Homework help starts here! Science Chemistry Q&A Library Q1:Drawand label the Cis-and Trans-isomers of the molecule represented by the following condensed formula. CH3CH=CHCH2CH3. Q1:Drawand label the Cis-and Trans-isomers of the molecule represented by the following condensed formula.
Cis and Trans Isomers - Chemistry Steps Cis and Trans Isomers You may also need to classify two molecules with a cis/trans double bond or a ring system. Because the connectivity of atoms is the same and the arrangement is different, these are stereoisomers. Specifically, because they are not mirror images, we classify them as diastereomers. So, cis and trans isomers are diastereomers.
Answered: Label the following as cis, trans, or… | bartleby 28. Transcribed Image Text: Label the following as cis, trans, or neither. CI to th CH3 CI H OCH3 H Blank 1 H₂N. Blank 2 H Blank 3 H CH3 CH3 CH₂CH3 Blank 4 Blank 5 OH Blank 6.
Cis Trans and E Z Geometric Isomers - Leah4sci 1) First, name the alkene using the tutorial linked below. 2) Then, simply add 'cis' or 'trans' in front of the name. Take the 2 geometric isomers of 2-butene: Their proper names are as follows: When there is only one pi bond, you don't have to specify which carbon is cis or trans since. It's self-understood.
Cis-trans isomerism - Wikipedia Cis-trans isomerism, also known as geometric isomerism or configurational isomerism, is a term used in chemistry that concerns the spatial arrangement of atoms within molecules. The prefixes "cis" and "trans" are from Latin: "this side of" and "the other side of", respectively.In the context of chemistry, cis indicates that the functional groups (substituents) are on the same side of some ...
The molecule dibromoethene (C2H2B12) has a cis and trans ... - Quesba The molecule dibromoethene (C2H2B12) has a cis and trans form. Draw these molecules and label them as polar or nonpolar. Why is one polar and the other nonpolar?
Label the molecules as cis or trans. - ZuoTi.Pro Draw and label both the cis and... 1. Name the molecules shown below Br Br 2. Draw and label both the cis and trans forms of 1-isopropyl-2-methylcyclobutane. draw the cis and trans isomers of 2-pntane and label them. b. draw trans -4,4 dimethyl-2-pentane...
SOLVED:a. Which of the following compounds can exist as cis-trans ... For those compounds that can exist as cis and trans isomers, draw and label the isomers. Answer (a) 1 and 3 can exits as cis-trans isomers. 2 and 4 cannot exist as cis and trans isomers because identical substituents are bonded to the same $\mathrm{sp}^{2}$ hybridized carbon. Upgrade to View Answer. Related Courses. Chemistry 102.
Study Chapter 4 Flashcards | Quizlet Label each pair of compounds below as: a. conformational isomers b. stereoisomers ... c. cis-trans isomers d. both b and c e. a, b and c ... a. identical molecules b. constitutional isomers c. stereoisomers d. different molecules. b. A: axial B: equatorial. Consider the two methyl groups indicated with letters in the following molecular model ...
Which of the following molecules can exist as cis and trans ... - OneClass 14. Which of the following molecules have NO cis/trans isomers? If there is a cis/trans isomer, draw and label both isomers as E and Z. 15. Give the mechanism for the following reaction and label the rate limiting step + H20 heat 16. Predict the major product of both reactions and label products as Hoffman or Zaitsev's product.
Geometric Isomerism Cis- and Trans- Mean in Chemistry - ThoughtCo In geometrical isomer nomenclature, the prefix cis- and trans- are used to identify which side of the double bond the similar atoms are found. The cis- prefix is from the Latin meaning "on this side". In this case, the chlorine atoms are on the same side of the carbon-carbon double bond. This isomer is called cis-1,2-dichloroethene.

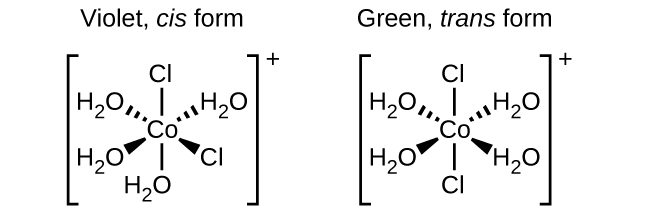










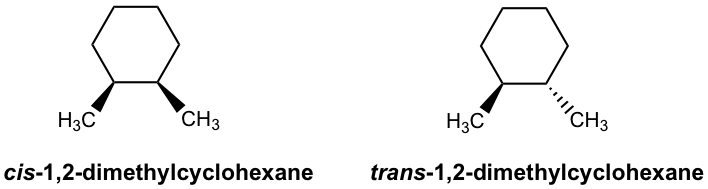


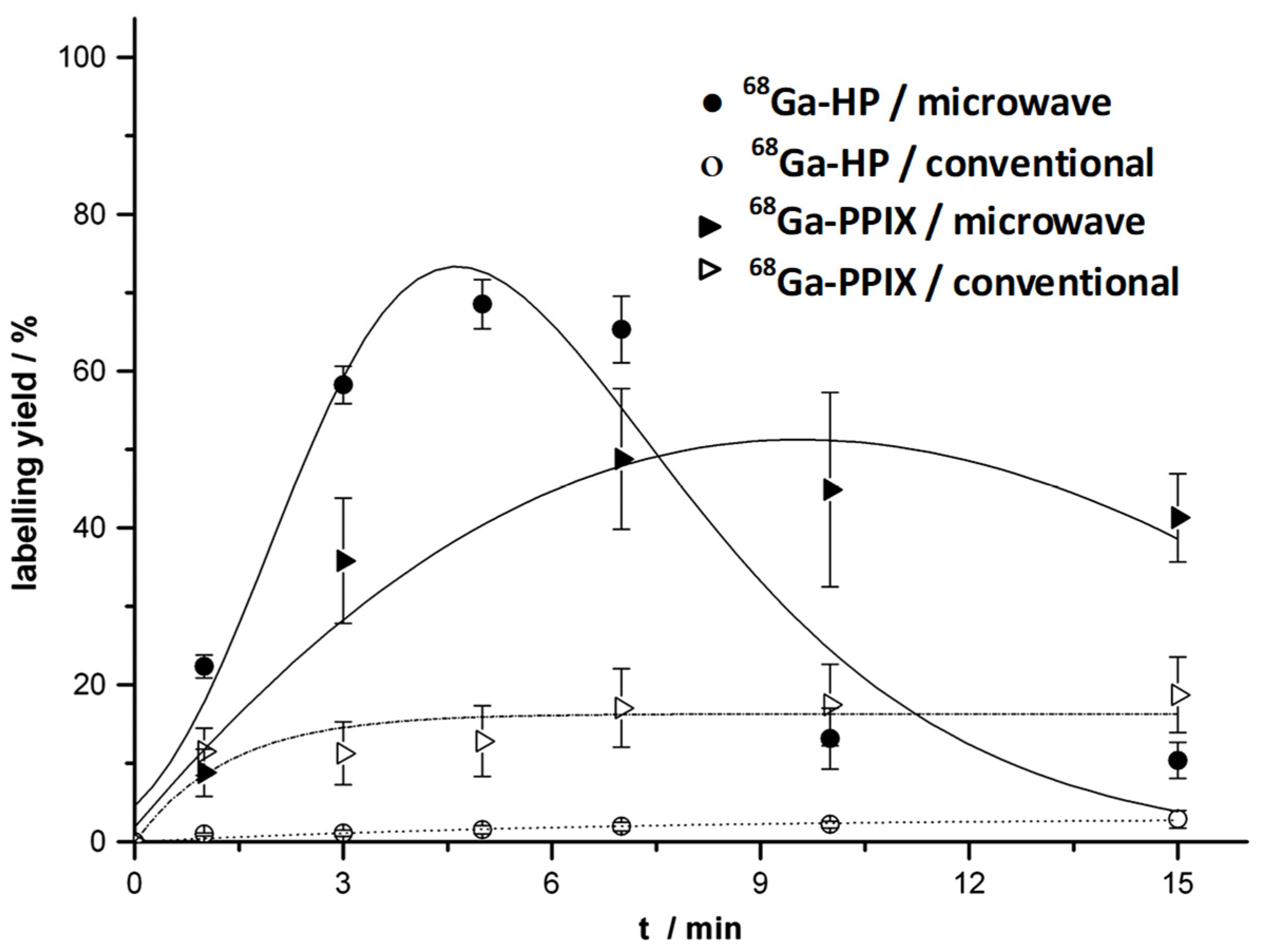

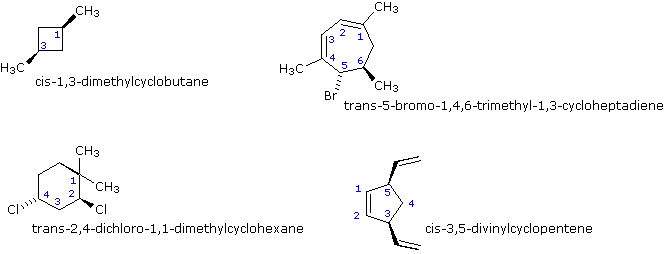




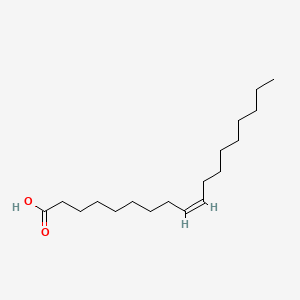
Post a Comment for "44 label the molecules as cis or trans."