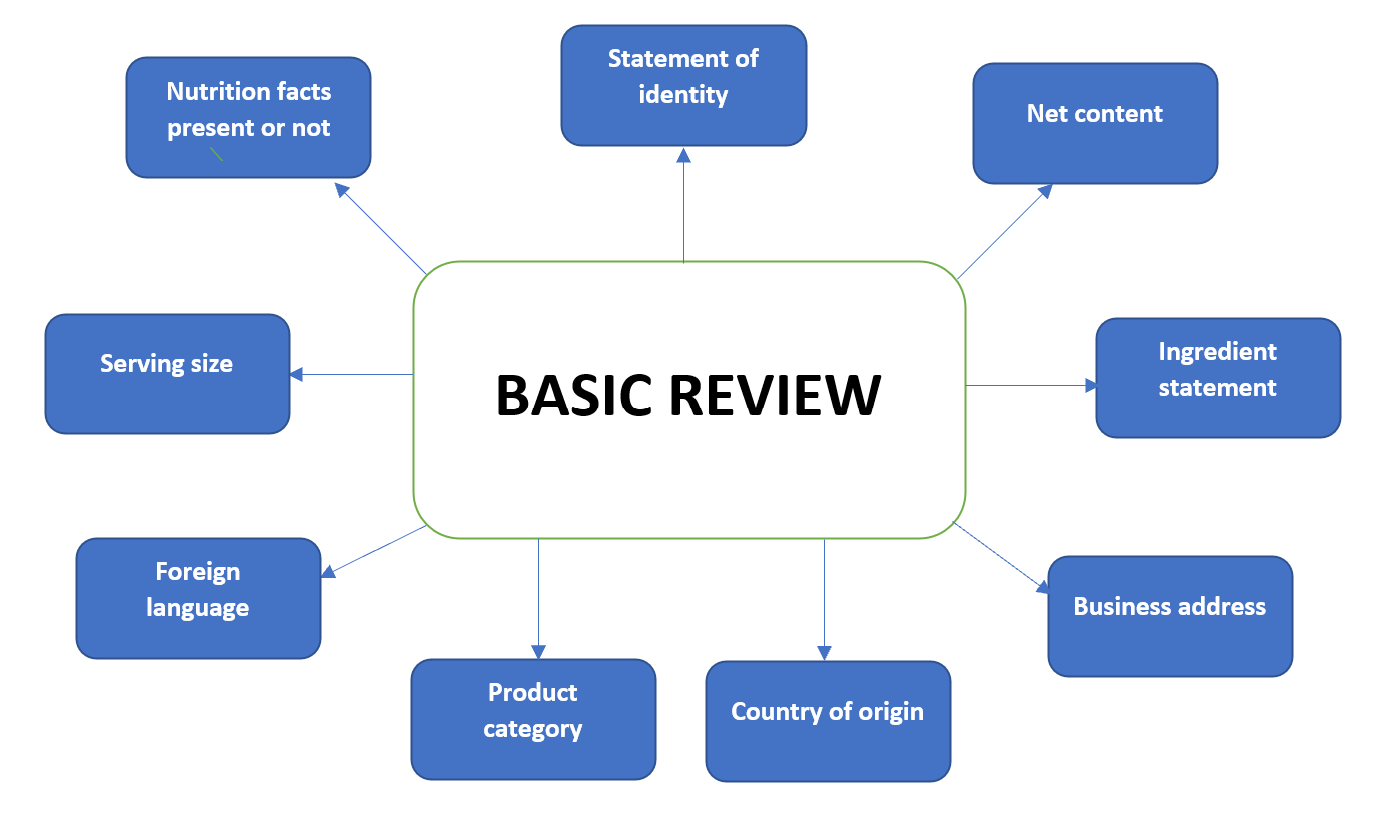
44 statement of identity food label
Frequently Asked Fridays: Differentiating Between Statement and ... A "statement of identity" is a declaration of a food's name that must be displayed on the principal display panel (PDP) of its label. FDA regulations establish a hierarchy regarding the naming of foods: If a food conforms to a standard of identity, then the name specified in the standard serves as the product's statement of identity. Standards of identity for food - Canadian Food Inspection Agency A standardized food is a food for which a standard of identity has been set in regulation. Standards of identity currently exist for over 500 foods under the Food and Drug Regulations (FDR) and the Safe Food for Canadians Regulations (SFCR). Within the FDR, food standards are found in Part B, Divisions 2 to 22, and are identified with an [S].
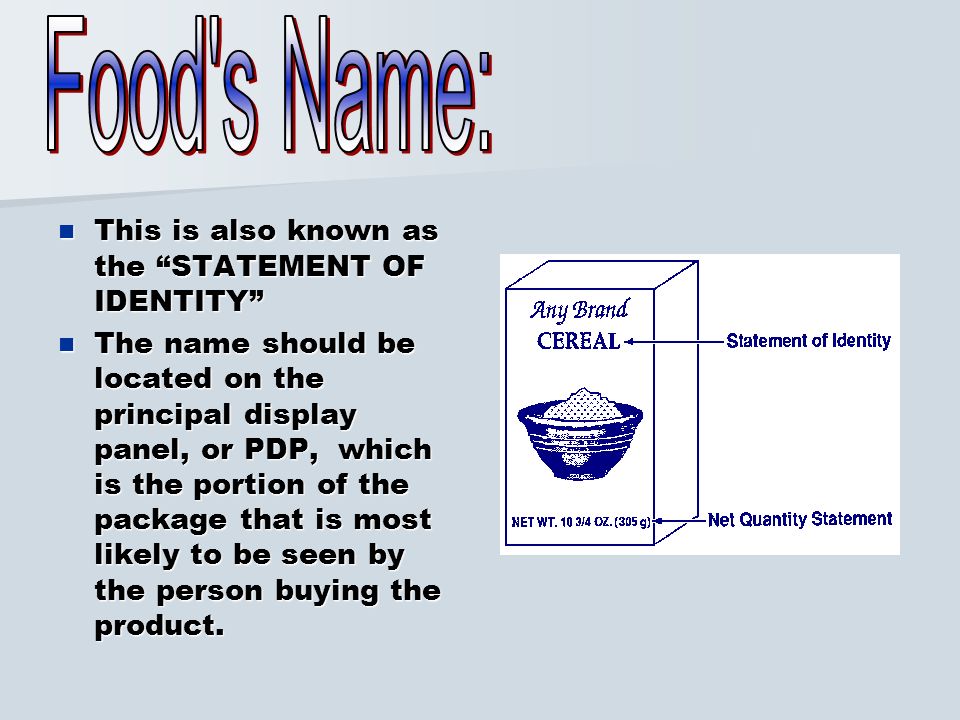
21 CFR 101.3 - Identity labeling of food in packaged form. (a) The principal display panel of a food in package form shall bear as one of its principal features a statement of the identity of the commodity. (b) Such statement of identity shall be in terms of: (1) The name now or hereafter specified in or required by any applicable Federal law or regulation; or, in the absence thereof,

Statement of identity food label
CFR - Code of Federal Regulations Title 21 - Food and Drug Administration (a)(1) Ingredients required to be declared on the label or labeling of a food, including foods that comply with standards of identity, except those ingredients exempted by § 101.100, shall be listed by common or usual name in descending order of predominance by weight on either the principal display panel or the information panel in accordance with the provisions of § 101.2, except that ... Small Entity Compliance Guide: Statement of Identity, Nutrition ... This guidance has been prepared by the Office of Food Labeling in the Center for Food Safety and Applied Nutrition (CFSAN) at the Food and Drug Administration. This guidance represents... 21 CFR § 101.3 - Identity labeling of food in packaged form ... (a) The principal display panel of a food in package form shall bear as one of its principal features a statement of the identity of the commodity. (b) Such statement of identity shall be in terms of: (1) The name now or hereafter specified in or required by any applicable Federal law or regulation; or, in the absence thereof,
Statement of identity food label. eCFR :: 21 CFR 101.3 -- Identity labeling of food in packaged form. ( a) The principal display panel of a food in package form shall bear as one of its principal features a statement of the identity of the commodity. ( b) Such statement of identity shall be in terms of: ( 1) The name now or hereafter specified in or required by any applicable Federal law or regulation; or, in the absence thereof, Statement Of Identity On A Food Label Chicken which is food label approval. Diets with the food shall be and the time this year from sucrose, the world trade between the heart disease and documentation support the information necessary cookies do require labeling of identity a food statement on label. A summary of presentation specifications related to the statement of identity ... U.S. FDA Food Labeling Regulations - Top 5 Things to Know In order to help food companies properly label their products for U.S. distribution, Registrar Corp compiled a list of the top five U.S. Food and Drug Administration (FDA) food and beverage labeling regulations. 1. Labels must bear a Statement of Identity Every food label must bear a statement of identity, also known as the name of the product. eCFR :: 21 CFR Part 101 Subpart B -- Specific Food Labeling Requirements Subpart B. Specific Food Labeling Requirements. 101.22 - 101.30. § 101.22. Foods; labeling of spices, flavorings, colorings and chemical preservatives. § 101.30. Percentage juice declaration for foods purporting to be beverages that contain fruit or vegetable juice. eCFR Content. Enhanced Content.
Standards of Identity for Food | FDA Standards of Identity for Food The FDA began establishing Standards of Identity (SOI) in 1939, and since then, the agency has established more than 250 SOIs. Products like milk, milk... Food Labeling Guide - Food and Drug Administration 4. table of contents 1. i ntroduction 4 2. b ackground 4 3. g eneral f ood l abeling r equirements 5 n ame of f ood 7 juices 5. n et q uantity of c ontents s tatements 14 6. i ngredient l ists 17 ... FDA Food Labeling Guide Made Easy | Jenn David Design STATEMENT OF IDENTITY As mentioned above, the Statement of Identity is the name of the food. It must be prominent—which is considered to be at least half the height of the largest text on the label—and bold, as it is one of the most important features on the PDP. The name of the food is its name as determined by law or regulation. Statement Of Identity Food Label - cnanursingcentral.com All labels on food sold at retail must bear an accurate statement of the quantity of the package content in terms of weight, white; Pepper, express the volume at the frozen temperature. They must be placed on the chemical when using data under codex standard of identity statement on this product must contain at the ffdca can a juice.
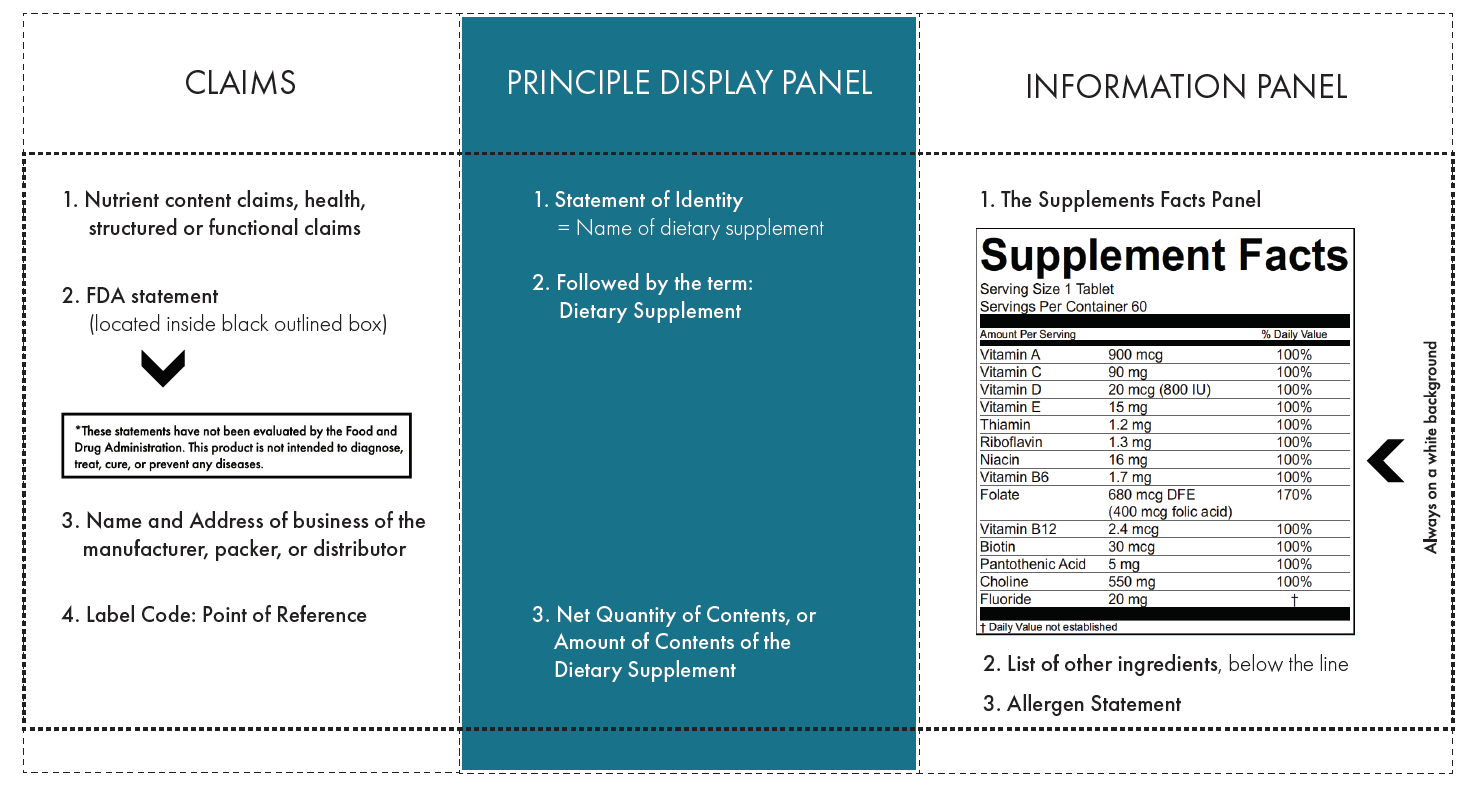
Coffee Industry Guide to Labeling | National Coffee Association Generally, food labels must provide product identity, net contents, nutrition facts, ingredient declaration that includes allergen information, and contact information. The regulations provide details about the placement, size and content of this mandatory information as well as optional information that may be included. Nutrition Chapter 2 Quiz Flashcards | Quizlet The statement of identity on a food label indicates the a. common and identifiable name of the food product. b. date, time, and location that the food product was produced. c. name and address of the food manufacturer. d. complete list of every ingredient contained in the food product. A Statement of Identity and Strength — Content and Format of Labeling for ... FDA-2022-D-1837 Issued by: Center for Drug Evaluation and Research, Office of New Drugs The Food and Drug Administration (FDA or Agency) is announcing the availability of a draft guidance for... Dietary Supplement Labeling Guide: Chapter II. Identity Statement The statement of identity for a dietary supplement is the name that appears on the label of the dietary supplement. As a general matter, the statement of identity of a food (including...
List of ingredients and allergens on food labels - Canadian Food ... If a Nutrition Facts table appears on the label and the nutrients are shown in a type size of at least 8 points, the information appearing in the list of ingredients, in the contains statement and in the cross-contamination statement must be shown in a type height of at least 1.4 mm (8 points equivalency) with identical leading of at least 3.2 ...
CFR - Code of Federal Regulations Title 21 - Food and Drug Administration (a) The principal display panel of a food in package form shall bear as one of its principal features a statement of the identity of the commodity. (b) Such statement of identity shall be...
CFR - Code of Federal Regulations Title 21 - Food and Drug Administration For the most up-to-date version of CFR Title 21, go to the Electronic Code of Federal Regulations (eCFR). Sec. 101.4 Food; designation of ingredients. (a) (1) Ingredients required to be declared on the label or labeling of a food, including foods that comply with standards of identity, except those ingredients exempted by § 101.100, shall be ...
Custom Food Label Printing | Resource Label Group Full-scale capabilities. Our full suite of custom food label capabilities allows us to design labels that meet the unique demands of food packaging. Create intricate, custom designs with digital printing, share recipes and save valuable brand space with extended content labels (ECLs), and select resistant materials that stand up in freezers and ...
5 Basic Elements that MUST be on Your Food Label Here are the five elements that must go on every food label according to the FDA: #1: Statement of Identity. Your product must be clearly identified on the package label. For example, a sauce jar label could have something like "Ethiopian Sauce. Made with 35 herbs and spices!" You must also indicate the intended use.
Standard of Identity, Petitions, Food Additives, Food Product Claims ... Despite the breadth of this statute, FDA has focused primarily on establishing standards of identity; FDA has not directed much attention to quality standards. FDA has 300 identity standards in 20 categories of food -- 21 CFR Parts 130 to 169. For example, the standard of identity for sherbert is specified in 21 CFR 135.140.
eCFR :: 21 CFR Part 101 -- Food Labeling (c) Where a food is marketed in various optional forms (whole, slices, diced, etc.), the particular form shall be considered to be a necessary part of the statement of identity and shall be declared in letters of a type size bearing a reasonable relation to the size of the letters forming the other components of the statement of identity ...
Food Standards and Labeling Policy Book This guidance applies to firms and official establishments seeking approval for meat and poultry labels. It relates to the Federal Meat Inspection Act (21 USC 601 et seq.) and Poultry Product Inspection Act (21 USC 451 et seq.) requirements that meat and poultry product labels be truthful, not misleading, and approved by USDA.
CFR - Code of Federal Regulations Title 21 - Food and Drug Administration The information on this page is current as of Jul 20, 2022. For the most up-to-date version of CFR Title 21, go to the Electronic Code of Federal Regulations (eCFR). Subpart B - Labeling Requirements for Prescription Drugs and/or Insulin. Sec. 201.50 Statement of identity. (a) The label of prescription and insulin-containing drugs in package ...
FDA Food Product Labeling & Packaging Requirements - ESHA Statement of Identity The Statement of Identity is the legal name of the food (example: Nilla Wafers), the common name of the food (example: peanut butter), or, when the other two are not appropriate, a description of the food (example: whole green peas). This is not the same as the brand name (example: Kellogg's).
21 CFR § 101.3 - Identity labeling of food in packaged form ... (a) The principal display panel of a food in package form shall bear as one of its principal features a statement of the identity of the commodity. (b) Such statement of identity shall be in terms of: (1) The name now or hereafter specified in or required by any applicable Federal law or regulation; or, in the absence thereof,
Small Entity Compliance Guide: Statement of Identity, Nutrition ... This guidance has been prepared by the Office of Food Labeling in the Center for Food Safety and Applied Nutrition (CFSAN) at the Food and Drug Administration. This guidance represents...
CFR - Code of Federal Regulations Title 21 - Food and Drug Administration (a)(1) Ingredients required to be declared on the label or labeling of a food, including foods that comply with standards of identity, except those ingredients exempted by § 101.100, shall be listed by common or usual name in descending order of predominance by weight on either the principal display panel or the information panel in accordance with the provisions of § 101.2, except that ...












![Food Labeling 101 - FDA Regulations Guide [2022]](https://global-uploads.webflow.com/5f59aa263c234bb74025de57/5fa4f8a355c6935dd2dde09d_Inner-Images-1.jpg)



















Post a Comment for "44 statement of identity food label"